Perisotin assay
Periostin is a matricellular protein which binds to ECM and cell surface receptors. It facilitates tissue remodeling and collagen crosslinking through its interaction with other ECM proteins and has been shown to induce myofibroblast differentiation, proliferation, expression of Collagen I/III and increase wound healing in the fibrosis setting. It has also been shown to regulate key aspects of tumour biology, including proliferation, invasion, matrix remodeling, and dissemination to pre-metastatic niches in distant organs. Myofibroblasts isolated from Dupuytren’s nodules spontaneously produce substantial amounts of this secreted protein and this may contribute to the chronicity of this disease driving the pro-fibrotic phenotype.
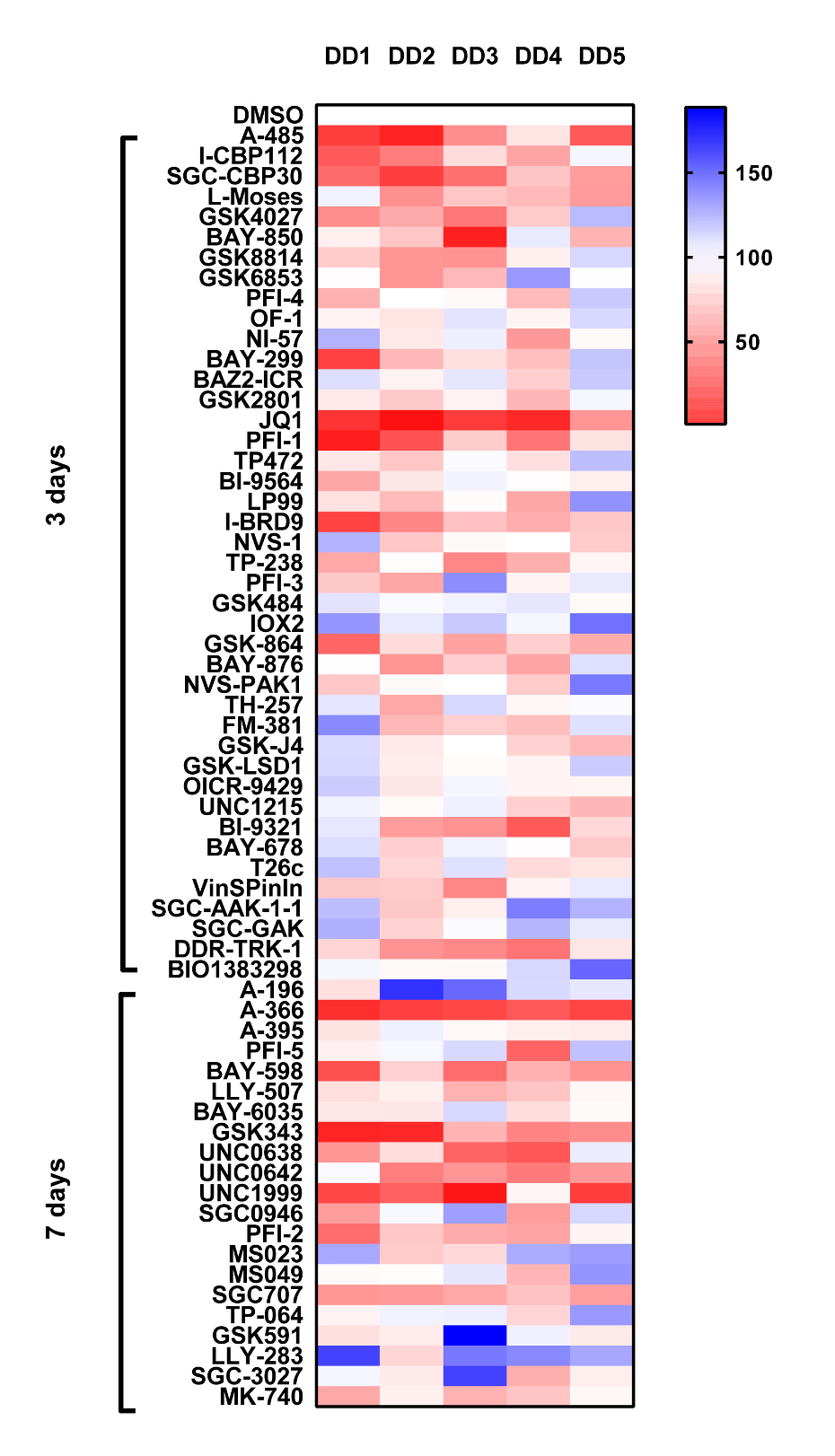
We have set up an assay to detect periostin secretion by ELISA. Using surgically excised tissue from Dupuytren’s patients we derived early passage myofibroblast2 (p2), Cells are allowed spread and adhere overnight then treated with 1µM of probe for 3 days or 7 days (MT) (cells are supplemented with a further dose on day 3). At the end of the assay period, cell supernatants harvested and perisotin levels are determined by ELISA.
Analysis from the screen highlights the bromodomain inhibitors JQ1, PFI-1, SGC-CBP30 and I-CBP112 all significantly inhibit perisotin expression. The PCAF inhibitor L-Moses and less consistently GSK4027 also inhibited perisotin expression. Significant inhibition was also observed with the MT probes A-366, GSK343, UNC0638, UNC642 and UNC1999. However given their strong anti-proliferative effects (as with JQ1/PFI-1), this maybe a reflection of there being fewer cells at the end of the 7 day assay period. An exception is the PRMT3 probe SGC-707 which displays no toxicity but does consistently inhibit perisotin expression.
- Surgically excised tissue from Dupuytren’s patients was treated with collagenase to derive single cell suspensions; cells were passaged (p2) and are termed myofibroblasts.
- Cells were seeded in 48 wells at 5 x103/well and left to adhere overnight. The following day probes were added at 1µM, using DMSO as the vehicle control. Probes were incubated with the cells for 3 days or in the case of methyltransferase (MT) probes 7 days (cells were treated with MT probes again at day 3).
- At the end of the assay period the concentration of perisotin in cell supernatants was determined by ELISA (R&D systems, Oxford, UK) according to the manufacturer’s instructions. Absorbance was read and analyzed at 450 nm on a spectrophotometric ELISA plate reader (Labsystems Multiskan Biochromatic) using Ascent software (version 2.4.2).
- Data is expressed as % compared to DMSO control (100%), DD1-5 Dupuytrens’s disease patients.
Periostin